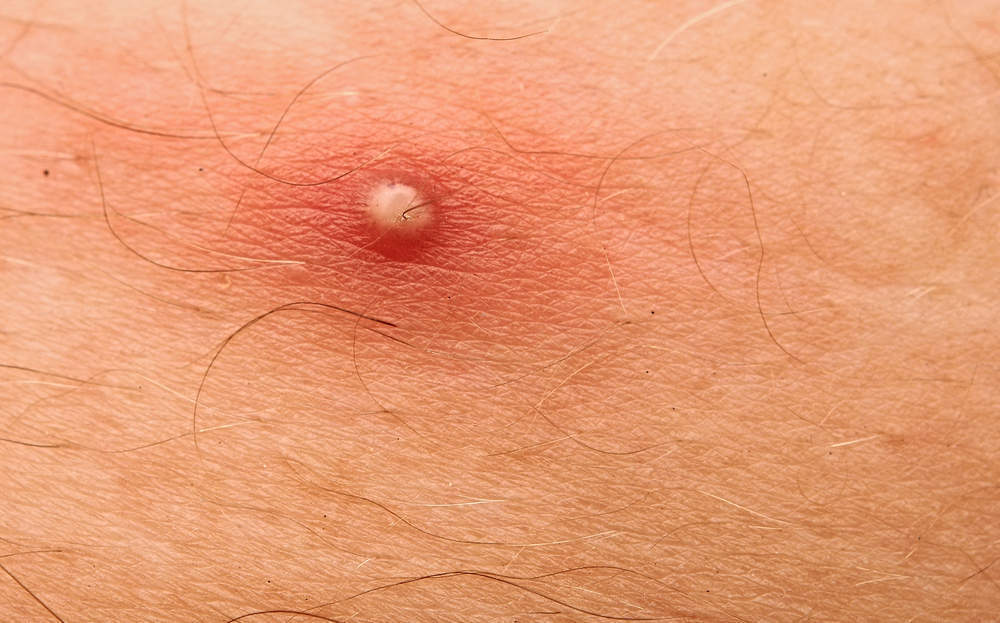
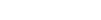In the US 56.4m people are believed to suffer from acne.
It affects more than twice as many people as psoriasis and atopic dermatitis combined – but while new and innovate products have revolutionised the treatment of these two conditions, developments in the treatment of acne have not managed to keep up.
The first biologic drugs for psoriasis were approved in 2003, and since then a number of biologics have made it to market.
In 2017, Dupixent became the first biologic to be approved for atopic dermatitis, and is expected to benefit patients whose disease is not adequately controlled with topical prescription therapies.
Biologics, unlike topical therapies, target the patient’s immune system directly, meaning they are are associated with fewer side effects and are more effective.
What next for acne?
Acne patients could potentially benefit in the near future from the approval of Dermia’s Olumacostat Glasaretil, a small molecule drug designed to target excessive sebum production.
US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThis is currently undergoing Phase III trials, the results of which are expected to be published in the first half of 2018.
If these trials are successful Dermira expects to start marketing the drug in 2019.
However, sufferers will need to wait a little longer for acne-treating biologics to reach the market.
But what about biologics?
It is possible that a biologic will be approved for acne at some point between now and 2023, but a date closer to the end of this period would be most likely.
There are currently 10 biologic products in the acne pipeline, but only three are in clinical development, one of which is at the final stage.
Acne patients are often reluctant to seek treatment at the moment, as current options are associated with poor effectiveness or severe adverse effects.
A company that can succeed in bringing a biologic to market that can effectively treat the condition while minimising side effects would be able to command high revenues, and capture a large share of the dermatology market.